Enzytec obtains Sanitary Permit for Importation and Distribution of Health Products according to RDC 16/2013 ANVISA
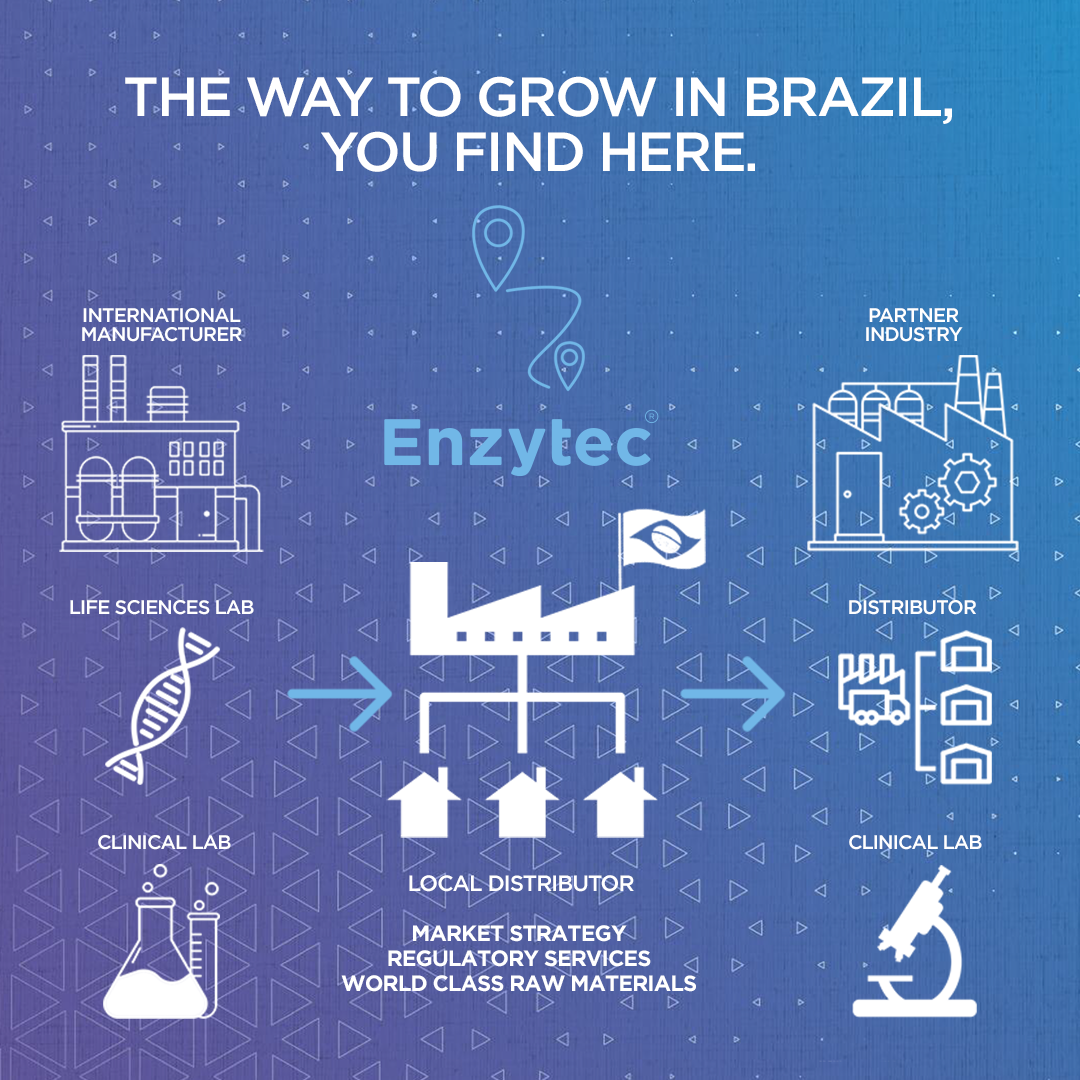
ENZYTEC NEWS Enzytec obtains Sanitary Permit for Importation and Distribution of Health Products according to RDC 16/2013 ANVISA In April 2022, Enzytec Biotecnologia obtained the Sanitary Permit for Importation and Distribution of Health Products. With long experience in regulatory consulting and technology development, in this new phase the company will also act as a distributor […]